 The concept of heat and temperature are studied together in science, which is somewhat related but not alike. The terms are very common, due to their wide usage in our day to day life. There exist a fine line which demarcates heat from temperature, in the sense that heat is thought of, as a form of energy, but the temperature is a measure of energy.
The concept of heat and temperature are studied together in science, which is somewhat related but not alike. The terms are very common, due to their wide usage in our day to day life. There exist a fine line which demarcates heat from temperature, in the sense that heat is thought of, as a form of energy, but the temperature is a measure of energy.
The fundamental difference between heat and temperature is slight but significant, heat is the overall energy of the molecular motion, whereas temperature is the average energy of the molecular motion. So, let’s take a look at the article given below, in which we have simplified the two for you.
Content: Heat Vs Temperature
Comparison Chart
| Basis for Comparison | Heat | Temperature |
|---|---|---|
| Meaning | Heat is the amount of energy in a body. | Temperature is the measure of the intensity of heat. |
| Measures | Total kinetic and potential energy contained by molecules in an object. | Average kinetic energy of molecules in a substance. |
| Property | Flows from hotter object to cooler object. | Rises when heated and falls when cooled. |
| Working ability | Yes | No |
| Unit of measurement | Joules | Kelvin |
| Device | Calorimeter | Thermometer |
| Labelled as | Q | T |
Definition of Heat
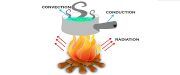
The heat of an object is the aggregate energy of all molecular movement inside the object. A form of energy that is transmitted from one object or source to another due to the differences in their temperature. It moves from a hotter object to the cooler one. Its measurement can be done in energy units, i.e. calorie or joules. The transfer of heat can take place in three ways, which are –
- Conduction: Heat transfer between molecules which are in direct contact with each other, without the movement of particles.
- Convection: The transfer of heat that takes place due to the movement of particles from one place to another is convection.
- Radiation: When the heat is transferred through a medium or vacuum, wherein space in between, is not heated up.
Definition of Temperature
Temperature is defined as the average kinetic energy of all molecules together, i.e. average energy of all the particles in an object. As an average measurement, the temperature of a substance does not rely on its size (number of particles) and type. It identifies how hot or cold an object is, in degrees. It also measures, the speed of atoms and molecules of the substance.
It can be measured in various scales, which are – Kelvin, Celsius and Fahrenheit. The thermometer is used to gauge the temperature of the object.
Key Differences Between Heat and Temperature
The differences between heat and temperature can be drawn clearly on the following grounds:
- Heat is nothing but the amount of energy in a body. As against this, temperature is something that measures the intensity of heat.
- Heat measures both kinetic and potential energy contained by molecules in an object. On the other hand, temperature measures average kinetic energy of molecules in substance.
- The main feature of heat is that it travels from hotter region to cooler region. Unlike temperature, which rises when heated and falls when cooled.
- Heat possesses the ability to work, but the temperature is used exclusively to gauge the extent of heat.
- The standard unit of measurement of heat is Joules, while that of temperature is Kelvin, but it can also be measured in Celsius and Fahrenheit.
- Calorimeter is a device, which is used to measure the heat. On the other hand, temperature can be measured by thermometer.
- Heat is represented by ‘Q’ whereas ‘T’ is used to represent temperature.
Conclusion
Both heat and temperature are the concepts of thermodynamics; that works together to let the energy flow from hotter body to the cooler body. While heat depends on the number of particles in an object, temperature does not depend on a number of particles in an object because it is an average measurement.





jo shmo says
Thank you so much
Saurabh says
Thanks 4 increased my iq
ROSHAN YADAV says
All answers is already right
Park Liza says
Thank for the help.
foot boy says
Thank u very much it was very help
Amy says
Thank you!
Nirupama Tiwari says
thank you very much
can we measure temperature using heat flux value?
cyril b mabanag says
thank you
Hajara says
Nice One
Ashwin says
it is good
Temidayo says
It helps me a lot with my project
sam says
Thank you so much for the extra help.
Wasiu mateen says
Good